 More than 15 years after the discovery of kidney injury molecule-1 (KIM-1) by Joseph Bonventre, MD, PhD, researchers in the HSCI Kidney Disease Program—of which Bonventre is a member—have found that the molecule promotes the scar tissue formation typically associated with chronic kidney disease, which affects approximately 9-10 percent of the world’s population. The finding was made in mice.
More than 15 years after the discovery of kidney injury molecule-1 (KIM-1) by Joseph Bonventre, MD, PhD, researchers in the HSCI Kidney Disease Program—of which Bonventre is a member—have found that the molecule promotes the scar tissue formation typically associated with chronic kidney disease, which affects approximately 9-10 percent of the world’s population. The finding was made in mice.
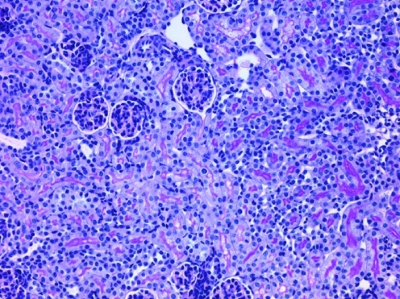
Scar tissue naturally forms in the kidney as it is affected by many disease states, including diabetes. A progressive buildup of scar tissue is called fibrosis, and continues to develop over time even if the original causes are treated. Fibrosis is exacerbated by hypertension and some medications, and is often seen with aging.
In response to progressive injury, kidney cells produce the molecule, KIM-1, which maintains its expression over time in chronic kidney disease, and has been known as an early predictive molecule and a useful biomarker for kidney injury in preclinical trials and in patients. What scientists have long wanted to find out, however, was what the molecule actually does in the kidney.
To isolate the function of KIM-1, HSCI researchers, led by Kidney Disease Program Head Leader Benjamin Humphreys, MD, PhD, and Bonventre, bred mice that expressed KIM-1 in kidney cells without any evidence of injury. Within four weeks, the mice showed kidney inflammation with fibrosis and other signs of kidney disease.
“This paper points to the fact that if KIM-1 is maintained in expression over time, it could have important maladaptive consequences, and could actually be a contributor to the progression of chronic kidney disease,” said Bonventre, who is Chief of the Renal Unit and Chief of the Bioengineering Division at Brigham and Women’s Hospital.
Looking at the cellular environment of these cells, the investigators found that KIM-1, which is normally a receptor molecule found on the kidney cell membrane, does act to protect cells, at first by reabsorbing cellular debris and toxic compounds that are present in the kidney after an acute injury. But then over time, when there are too many toxic compounds, the constant reabsorption increases damage in the kidney cells and leads the kidney cells to produce molecules, which lead to the generation of fibrosis. “If we can block KIM-1 function in the setting of chronic injury, this could be a very effective therapeutic strategy,” said Humphreys.
“Fibrosis is a major unmet need in the pharmaceutical industry, because there’s no current therapies to slow down the progression,” Bonventre said. “What this paper points to is that KIM-1 may be a target for the development of therapeutic agents, and could slow chronic kidney disease progression. “
The research was supported by the Harvard Stem Cell Institute, the National Institutes of Health, the American Heart Association, the National Institutes of Diabetes and Digestive and Kidney Diseases, the China Scholarship Council-Harvard University Exchange, and the Deutsche Forschungsgemeinschaft.
Research Cited: Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. The Journal of Clinical Investigation. August 27, 2013
Photo: Kidney fibrosis (Credit: Bonventre et. al./JCI)
