Giant step toward new diabetes treatment
Harvard stem cell researchers today announced that they have made a giant leap forward in the quest to find a truly effective treatment for type 1 diabetes, a condition that affects an estimated 3 million Americans at a cost of about $15 billion annually:
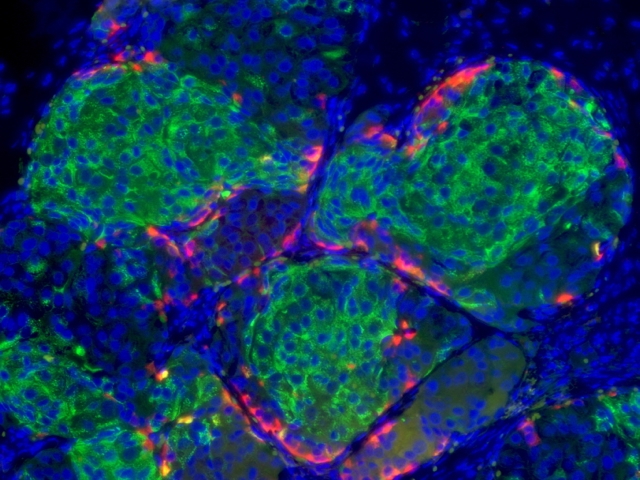
With human embryonic stem cells as a starting point, the scientists are for the first time able to produce, in the kind of massive quantities needed for cell transplantation and pharmaceutical purposes, human insulin-producing beta cells equivalent in most every way to normally functioning beta cells.
Doug Melton, who led the work and who 23 years ago, when his then infant son Sam was diagnosed with type 1 diabetes, dedicated his career to finding a cure for the disease, said he hopes to have human transplantation trials using the cells to be underway within a few years.
“We are now just one pre-clinical step away from the finish line,” said Melton, whose daughter Emma also has type 1 diabetes.
A report on the new work has today been published by the journal Cell.
Felicia W. Pagliuca, Jeff Millman, and Mads Gurtler of Melton’s lab are co-first authors on the Cell paper. The research group and paper authors include a Harvard undergraduate.
“You never know for sure that something like this is going to work until you’ve tested it numerous ways,” said Melton, Harvard’s Xander University Professor and a Howard Hughes Medical Institute Investigator. “We’ve given these cells three separate challenges with glucose in mice and they’ve responded appropriately; that was really exciting.
“It was gratifying to know that we could do something that we always thought was possible,” he continued, “but many people felt it wouldn’t work. If we had shown this was not possible, then I would have had to give up on this whole approach. Now I’m really energized.”
The stem cell-derived beta cells are presently undergoing trials in animal models, including non-human primates, Melton said.
Elaine Fuchs, the Rebecca C. Lancefield Professor at Rockefeller University, and a Howard Hughes Medical Institute Investigator who is not involved in the work, hailed it as “one of the most important advances to date in the stem cell field, and I join the many people throughout the world in applauding my colleague for this remarkable achievement.
“For decades, researchers have tried to generate human pancreatic beta cells that could be cultured and passaged long term under conditions where they produce insulin. Melton and his colleagues have now overcome this hurdle and opened the door for drug discovery and transplantation therapy in diabetes,” Fuchs said.
And Jose Oberholzer, MD, Associate Professor of Surgery, Endocrinology and Diabetes, and Bioengineering at the University of Illinois at Chicago, and its Director of the Islet and Pancreas Transplant Program and the Chief of the Division of Transplantation, said work described in today’s Cell “will leave a dent in the history of diabetes. Doug Melton has put in a life-time of hard work in finding a way of generating human islet cells in vitro. He made it. This is a phenomenal accomplishment.”
 Melton, co-scientific director of the Harvard Stem Cell Institute, and the University’s Department of Stem Cell and Regenerative Biology – both of which were created more than a decade after he began his quest – said that when he told his son and daughter they were surprisingly calm. “I think like all kids, they always assumed that if I said I’d do this, I’d do it,” he said with a self-deprecating grin.
Melton, co-scientific director of the Harvard Stem Cell Institute, and the University’s Department of Stem Cell and Regenerative Biology – both of which were created more than a decade after he began his quest – said that when he told his son and daughter they were surprisingly calm. “I think like all kids, they always assumed that if I said I’d do this, I’d do it,” he said with a self-deprecating grin.
Type 1 diabetes is an autoimmune metabolic condition in which the body kills off all the pancreatic beta cells that produce the insulin needed for glucose regulation in the body. Thus the final pre-clinical step in the development of a treatment involves protecting from immune system attack the approximately 150 million cells that would have to be transplanted into each patient being treated. Melton is collaborating on the development of an implantation device to protect the cells with Daniel G. Anderson, the Samuel A. Goldblith Professor of Applied Biology, Associate Professor in the Department of Chemical Engineering, the Institute of Medical Engineering and Science, and the Koch Institute at MIT.
Melton said that the device Anderson and his colleagues at MIT are currently testing has thus far protected beta cells implanted in mice from immune attack for many months. “They are still producing insulin,” Melton said.
Cell transplantation as a treatment for diabetes is still essentially experimental, uses cells from cadavers, requires the use of powerful immunosuppressive drugs, and has been available to only a very small number of patients.
MIT’s Anderson said the new work by Melton’s lab is “an incredibly important advance for diabetes. There is no question that ability to generate glucose-responsive, human beta cells through controlled differentiation of stem cells will accelerate the development of new therapeutics. In particular, this advance opens to doors to an essentially limitless supply of tissue for diabetic patients awaiting cell therapy."
Richard A. Insel, MD, chief scientific officer of the JDRF, a funder of Melton’s work, said the “JDRF is thrilled with this advancement toward large scale production of mature, functional human beta cells by Dr. Melton and his team. This significant accomplishment has the potential to serve as a cell source for islet replacement in people with type 1 diabetes and may provide a resource for discovery of beta cell therapies that promote survival or regeneration of beta cells and development of screening biomarkers to monitor beta cell health and survival to guide therapeutic strategies for all stages of the disease.”
Melton expressed gratitude to both the Juvenile Diabetes Research Foundation and the Helmsley Charitable Trust, saying “their support has been, and continues to be essential. I also need to thank Howard and Stella Heffron, whose faith in our vision got this work underway, and helped get us where we are today.”
While diabetics can keep their glucose metabolism under general control by injecting insulin multiple times a day, that does not provide the kind of exquisite fine tuning necessary to properly control metabolism, and that lack of control leads to devastating complications from blindness to loss of limbs.
About 10 percent of the more than 26 million Americans living with type 2 diabetes are also dependent upon insulin injections, and would presumably be candidates for beta cell transplants, Melton said.
“There have been previous reports of other labs deriving beta cell types from stem cells, no other group has produced mature beta cells as suitable for use in patients,” he said. “The biggest hurdle has been to get to glucose sensing, insulin-secreting beta cells, and that’s what our group has done.”
In addition to the institutions and individual cited above, the work was funded by the Harvard Stem Cell Institute, the National Institutes of Health, and the JPB Foundation.
Cited: Pagliuca, F., Millman, J. and Gürtler, M, et. al. Generation of functional human pancreatic beta cells in vitro. Cell. October 9, 2014.
Dr. Melton has made an author's proof available. Click here to download the PDF.
The beginning shows a spinner flask containing red culture media and cells, the cells being too small to see. Inside the flask you can see a magnetic stir bar and the flask is being placed on top of a magnetic stirrer.
This is followed by a time course series of images, magnified, showing how cells tart of as single cells and then grow very quickly into clusters over the next few days. The size of the clusters is the same as the size of human islets at the end.
The final image shows 6 flasks, enough for 6 patients, spinning away. If you look closely, you can see particles spinning around, the white dust or dots are clusters of cells, each containing about 1000 cells.
