Method for growing kidney organoids under flow enhances their vascularization and maturation, increasing their potential for drug testing and regenerative medicine
Mini-organs, called organoids, are grown in a culture dish. They contain many of the cell types and complex microarchitectures found in human organs, such as the kidney, intestine, and brain. Organoids have the potential to transform drug discovery, allowing researchers to experiment on samples of human tissue grown directly from patients.
The challenge
Because organoids are grown outside of a body, they lack the blood vessel structure, or vasculature, needed to circulate oxygen and nutrients, remove waste, and send messages between different cell types. This has been a major roadblock in maturing groups of cells in a dish into truly functional tissues.
For kidney organoids, this shortcoming has prevented researchers from emulating key kidney functions, such as blood filtration, reabsorption, and urine production. Stem cell scientists have been working to create vascularized kidney organoids that are robust enough to enhance renal drug toxicity testing and, ultimately, lead to new building blocks for renal replacement therapies.
A new approach, published in Nature Methods, demonstrates how organoids can be vascularized, opening the door to a flood of possibilities in stem cell research.
What the researchers found
Led by Jennifer Lewis and Ryuji Morizane, a team of scientists at the Harvard Stem Cell Institute (HSCI), the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), Brigham and Women’s Hospital, and the Wyss Institute for Biologically Inspired Engineering has developed a powerful new way to maintain and grow organoids.
The bioengineering approach exposes stem cell-derived organoids to fluidic shear stress — that is, the frictional force of flowing biological fluids. Using this technique, researchers were able to expand organoid-derived vascular networks significantly. Compared with previous, static culturing methods, the exposure improved the maturation of kidney compartments.
The quest for better kidney organoids
In 2015, HSCI faculty members Ryuji Morizane and Joseph Bonventre developed a method that enabled them to derive 3D kidney organoids from human pluripotent stem cells.
“While our organoids and those generated in other laboratories contained large numbers of well-organized nephrons and primitive blood vessels, they still lacked pervasive vascular compartments with perfusable lumens,” said co-corresponding author Morizane, M.D., Ph.D., Assistant Professor at Brigham and Women’s Hospital and Harvard Medical School.
More recently, researchers around the world have matured kidney organoids by implanting them into animals, where they can connect directly to the host’s vasculature.
“For the first time, our study demonstrates that by exposing growing organoids to fluid flow, a mechanical cue known to play an important role for tissue development in the body, we can greatly enhance their vascularization and maturation in vitro,” said Morizane.
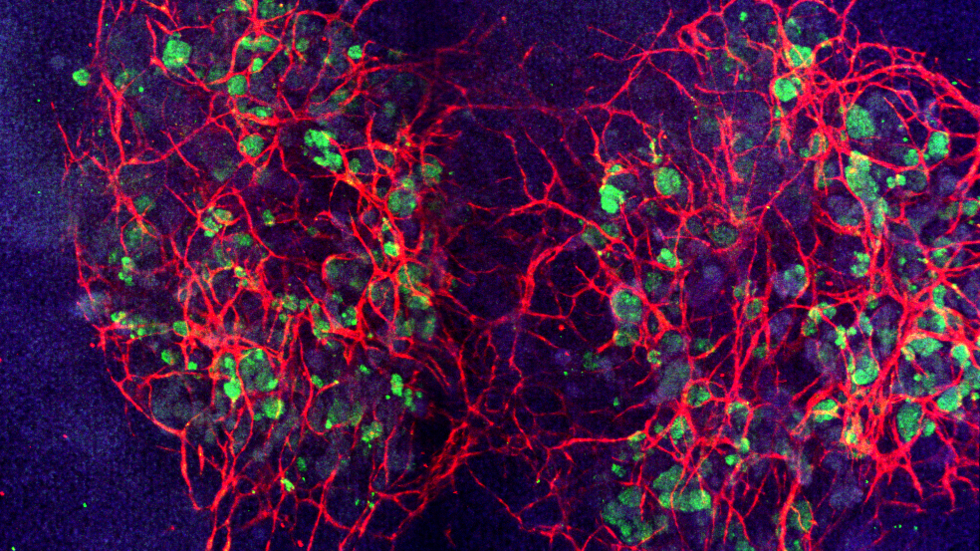
How they did it
To accomplish this feat, the team used expertise from the Lewis lab that has pioneered strategies to create vascularized human tissues, including 3D kidney-on-chip models. These strategies use 3D bioprinting that can be perfused and sustained for long durations. Building on this work, the team explored the idea that fluid flow could also promote the formation of blood vessels from precursor endothelial cells found in growing kidney organoids.
“We determined the right combination of underlying extracellular matrix, media additives, and fluidic shear stress under which human stem-cell derived organoids would flourish when grown in our 3D-printed millifluidic chips,” said Kimberly Homan, Ph.D., who is a co-first author on the study with Navin Gupta, M.D.
“The vascular networks form close to the epithelial structures that build the glomerular and tubular compartments, and in turn promote epithelial maturation. This integrated process works really like a two-way street,” added Gupta.
Homan is a Research Associate in Lewis’ group at the Wyss Institute and SEAS, and Gupta is a Clinical Research Fellow working on Morizane’s team at the Brigham.
Biological network on a chip
The vessels growing on the 3D-printed chips formed an interconnected network with open lumens. The network could be perfused with fluids, as confirmed by directly imaging fluorescent beads moving freely through them.
“We were excited to see that these vascularized glomerular and tubular structures develop through some of the same stages that nephrons experience during normal kidney development in vivo,” said Homan.
Why it matters
“This important advance opens up new avenues for accurately testing drug toxicity in vitro in differentiated nephron compartments and modeling kidney diseases, like polycystic kidney disease, that affect specific structures and cell types using patient-derived stem cells as the starting point,” said Lewis, Sc.D., whose work is truly interdisciplinary. She is a Core Faculty member of the Wyss Institute and co-leader of its 3D Organ Engineering Initiative, the Hansjörg Wyss Professor of Biologically Inspired Engineering at SEAS, and a member of the Harvard Stem Cell Institute.
“Our method may pave the way to also vascularize other types of organoids, such as the liver organoids,” she said.
Funding
The study was funded by the National Institutes of Health, Harvard’s Wyss Institute for Biologically Inspired Engineering, and the Harvard Stem Cell Institute.
Read more
The original version of this story can be found on the Wyss Institute website.
Source publication: Homan, K. A. and Gupta, N. et al. (2019). Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nature Methods. DOI: 10.1038/s41592-019-0325-y
