Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a leading cause of sudden death from cardiac arrest in children and young adults. Marked by racing, irregular heartbeats, the inherited arrhythmia is typically silent until a child suddenly collapses and faints, at an average age of 12. The trigger is an adrenaline surge, caused by exercise or emotional stress.
In a pair of new studies published in Circulation, researchers at the Harvard Stem Cell Institute (HSCI) and Boston Children’s Hospital (BCH) report creating the first human tissue model of CPVT, replicating two patients’ abnormal heart rhythms, and then suppressing the arrhythmia with gene therapy in a mouse model. The work opens the possibility of genetically correcting CPVT and other inherited arrhythmias, and perhaps more common arrhythmias like atrial fibrillation.
“Our hope is to give gene therapy in a single dose that would work indefinitely,” said Vassilios Bezzerides, M.D., Ph.D., a BCH cardiologist who was involved in both studies. “Our work provides proof-of-concept for a translatable gene therapy strategy.”
Improving treatment options for CPVT
Current treatment for CPVT consists of drugs such as beta-blockers and flecainide, surgery to disconnect nerves innervating the left side of the heart, and an implanted cardioverter-defibrillator (ICD). But these approaches often don’t work, and ICDs can actually be dangerous in CPVT, inducing a life-threatening “electrical storm.” Often, children’s main option is simply avoiding physical and emotional exertion.
“Treatments for CPVT are currently pretty inadequate,” said Bezzerides. “Twenty-five to 30 percent of patients will have recurrent life-threatening arrhythmias despite treatment.”
HSCI faculty members William T. Pu, M.D., and Kevin Kit Parker, Ph.D. were co-senior authors on the first study, which aimed to further our understanding of how CPVT works. The researchers set out to build tissue models that faithfully replicated CPVT in a dish, using blood samples from two patients.
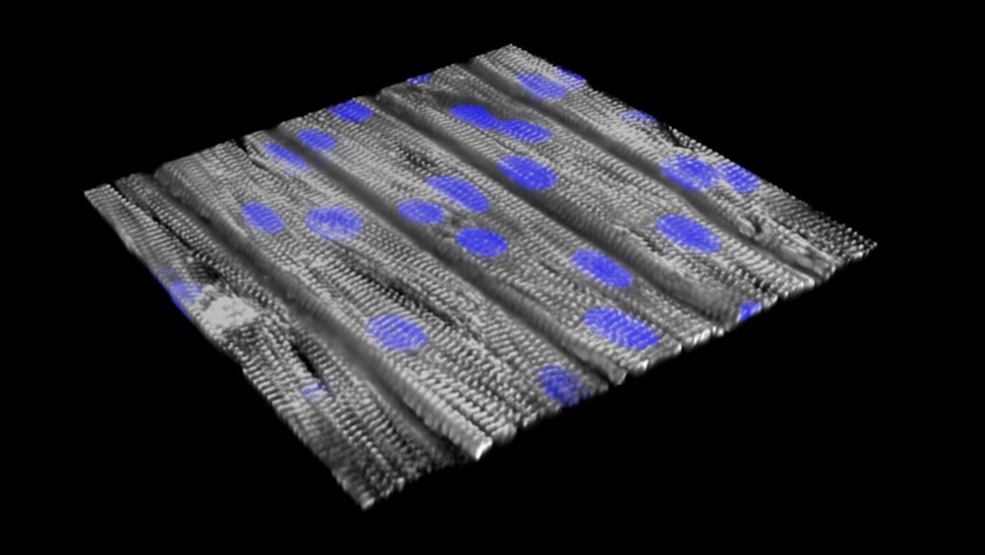
Building arrhythmic tissue
Both patients had a mutation in RYR2, the gene linked to most cases of CPVT. RYR2 encodes a channel that enables cells to release calcium — the first step in initiating a heart contraction. The scientists first reprogrammed their blood cells to become induced pluripotent stem (iPS) cells, capable of making virtually all cell types. From these, they made cardiomyocytes (heart muscle cells) carrying the CPVT mutations, and seeded these cells onto an engineered surface.
“The cells lined up in a specific direction similar to how heart muscle is organized,” said Pu, who directs Basic and Translational Cardiovascular Research at Boston Children’s. “The cells have very abnormal beating individually, but after assembly into tissue, they beat together, better modeling the actual disease. That’s why tissue-level models are important.”
Exercise test in a dish
The team next created an “exercise test in a dish.” They applied blue light to one end of the tissue, activating the cells and creating an impulse that moved along the sheet of cells to produce a contraction. To simulate exercise, they added the drug isoproterenol (similar to the stress hormone adrenaline) and applied infrared light to initiate faster heartbeats.
This testing helped reveal CPVT’s underlying mechanisms. When healthy heart tissue underwent the exercise test, calcium moved through the tissue in even waves. But in tissue models made from the patients with CPVT, the calcium waves moved at varying speeds, and in some parts of the tissue not at all. This resulted in an abnormal circular motion known as re-entry, much like what happens in the actual disease.
“When we paced the cells faster, the CPVT tissue sustained re-entrant arrhythmias, whereas normal tissue could handle it fine,” said Pu.
Finding a therapeutic target
Why does stress make CPVT patients vulnerable to life-threatening arrhythmias? To find out, Pu, Parker, and colleagues identified signaling molecules that are activated by adrenaline. They then used drugs and genome editing to selectively inhibit or modify them.
Through this strategy, they found that an enzyme called CaM kinase (CaMKII) chemically modifies RYR2, triggering cardiomyocytes to release more calcium. In healthy heart tissue, this causes no problem, but in CPVT, this modification combines with the inherited RYR2 mutation to produce excessive calcium levels in cells, precipitating arrhythmias.
“Nature designed CaMKII as part of the fight-or-flight response,” said Pu. “When you get excited, you release more calcium so the heart can beat faster. But when RYR2 is mutated, the channel is leaky, so the cell releases way too much calcium, which causes arrhythmia.”
When the researchers blocked the CaMKII modification, they eliminated the arrhythmias in the CPVT tissue model. They got the same effect when they blocked CaMKII itself with a potent and selective inhibitor, a peptide called AIP.
“The coupling of iPS technology and ‘organs on chips’ offers new opportunities for studies in precision medicine,” said Parker. “By replicating the patient’s disease in vitro, we can test candidate therapies and measure safety and efficacy, so that the right patients get tested with the right drug.
Inhibiting CPVT with gene therapy
In a separate study, a team led by Bezzerides tested a gene therapy approach to inhibiting CaMKII in a mouse model of CPVT. Because CaMKII acts on many tissues beside the heart — in fact, it’s needed by the brain to form memories — the team wanted to be able to inhibit the enzyme only in the heart.
They engineered a special virus which, injected into mice, selectively travelled to the heart and delivered the AIP gene. Testing found AIP in about 50 percent of heart cells, enough to suppress arrhythmias, but not in other tissues, including the brain.
Pu, senior author on the paper, plans to refine the gene therapy strategy together with Bezzerides and test it in a large animal model and eventually in patients with CPVT.
A general approach to heart disease
Bezzerides and Pu believe gene therapy could be effective for patients with CPVT caused by a variety of RYR2 mutations — more than 160 mutations have been reported. And they believe their overall strategy of inhibiting CaMKII in the heart could help treat more common causes of heart disease.
“CaMKII is not needed for normal heart function, but it becomes activated in many forms of heart disease,” said Pu. “In mouse models of many forms of heart disease, such as ischemic cardiomyopathy, atrial fibrillation, or hypertrophic cardiomyopathy, chronic CaMKII activation is detrimental. It is possible that our gene therapy approach to CaMKII inhibition could improve outcomes in these other types of heart disease.”
Discover more
The original version of this story was published on the Boston Children’s Hospital website on July 17, 2019.
Source article 1: Park, S.-J. and Zhang, D. et al. (2019). Insights into the Pathogenesis of Catecholaminergic Polymorphic Ventricular Tachycardia from Engineered Human Heart Tissue. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.119.039711
Source article 2: Bezzerides, V. J. et al. (2019). Gene Therapy for Catecholaminergic Polymorphic Ventricular Tachycardia by Inhibition of Ca2+/Calmodulin-Dependent Kinase II. Circulation. https://doi.org/10.1161/CIRCULATIONAHA.118.038514
