 Harvard stem cell scientists have found that cells known primarily for tempering immune response also exist in injured muscle tissue, an unexpected role for regulatory T cells.
Harvard stem cell scientists have found that cells known primarily for tempering immune response also exist in injured muscle tissue, an unexpected role for regulatory T cells.
Regulatory T cells, or Tregs for short, accumulate in the skeletal muscles of mice after injury or after the animals developed muscular dystrophy, the researchers found. Their discovery adds another surprising role to these immune cells’ repertoire, provoking interest in their potential for repairing wounds and perhaps even treating muscular dystrophy.
Dalia Burzyn, a postdoctoral fellow in the lab of HSCI Principal Faculty member Diane Mathis and HSCI Affiliated Faculty member Christophe Benoist, Morton Grove-Rasmussen Professors of Immunohematology, and colleagues showed that a unique population of Tregs promotes muscle repair by controlling both immune and nonimmune cells. Their report appears in the December 5 issue of Cell.
 Tregs typically keep the immune response in check after other immune cells have defeated pathogens such as viruses or bacteria. They also calm allergic reactions and dampen responses to tumors.
Tregs typically keep the immune response in check after other immune cells have defeated pathogens such as viruses or bacteria. They also calm allergic reactions and dampen responses to tumors.
In 2009 Mathis and her colleagues were the first to report an unusual, tissue-specific role for Tregs. They showed that Tregs found in fat tissue control insulin resistance and glucose intolerance, two important functions in metabolism.
Looking further, Burzyn and her colleagues turned their attention to Tregs found in skeletal muscle. In mice, these unique Tregs appear within days after injury but persist long after inflammation has subsided. Tests to compare gene activity showed these cells produced proteins different from those made by Tregs found in other tissues. Most striking was the abundance of a protein that boosts muscle repair.
These cells behaved the same way, whether the mice had been injured or whether they carried genetic mutations that cause muscular dystrophy and its chronic muscle damage.
“The most surprising and interesting thing we found was that Tregs are necessary for a proper muscle regeneration process,” Burzyn said. “We think Tregs promote an anti-inflammatory environment in the muscle and we also think they control other cells.”
Those other cells include stem cells, the researchers found when they collaborated with HSCI Executive Committee member Amy Wagers, Harvard Professor of Stem Cell and Regenerative Biology. Muscle stem cells are required to repair and regenerate muscle tissue.
“If we take muscle stem cells from mice that have been injured in the absence of these Tregs, the muscle stem cells cannot proliferate properly,” Burzyn said, describing experiments conducted in test tubes.
“The fact that these Tregs could actually affect the operation of muscle stem cells—that’s the part that’s really unexpected,” Mathis said.
A next step might be to compare muscle tissue from people with muscular dystrophy with tissue from mouse models of the disease.
Another potentially promising avenue stems from antigen receptor sequencing performed as part of the experiments. There are hints that the Tregs may be responding to an antigen—a harmful substance that spurs the production of antibodies—in injured muscle. Previous work with Tregs in fat tissue also suggested the presence of an antigen, Mathis said.
“This may be a whole new class of antigens that are important for keeping the tissues stable,” she said. “It’s a fascinating result.”
If so, the antigens could become a target for boosting this previously unknown function of Tregs.
“Immunology takes you to a lot of places you didn’t know you were going to go,” Mathis said.
This work benefited from public data generated by the Immunological Genome Project and was funded by the National Institutes of Health.
Story made available by Harvard Medical School. The original story was written by Elizabeth Cooney.
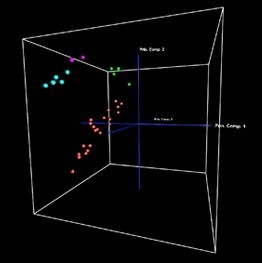
Photo: According to genome-wide expression analysis, different Treg populations are distributed in space according to their similarity: The position of the novel muscle Treg cells indicates that they are a unique population, more similar to the recently described fat Treg cells than to the most-studied lymphoid tissue Tregs. (Credit: Mathis/Benoist Lab)
