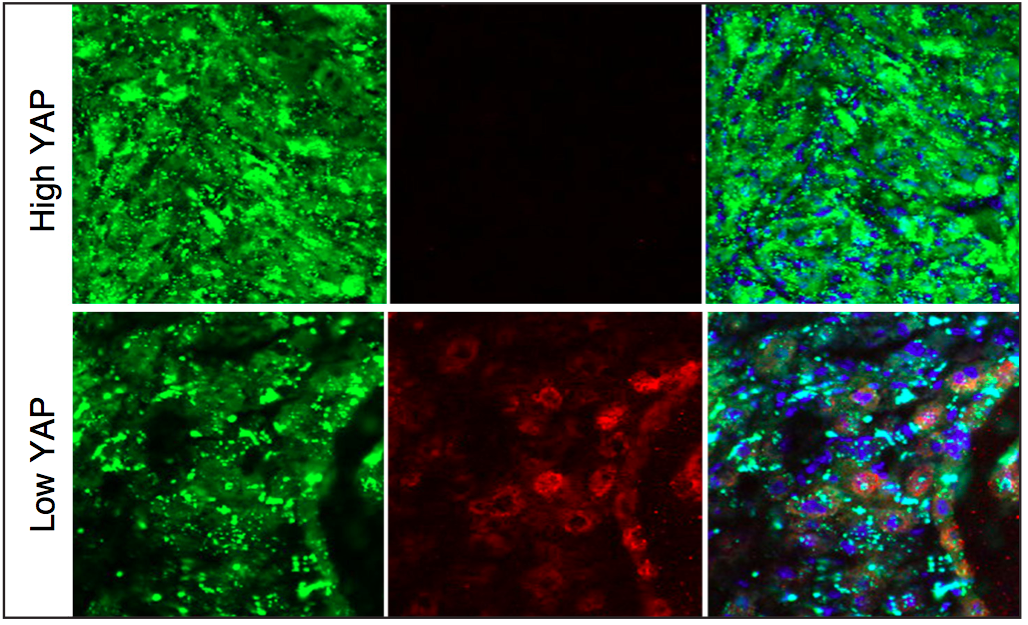
Mouse rhabdomyosarcoma tumors produced by activated muscle stem cells with high Yap activity (green; top panels) decrease in size when Yap activity is lowered (bottom panels, as shown by the green tumor cells starting to form muscle tissue and express normal muscle differentiation marker in red at the core of the regressing tumor). (Credit: Annie Tremblay, PhD)
Finding unlocks new therapeutic strategy for embryonal rhabdomyosarcoma
A collaborative study led by Harvard Stem Cell Institute scientists at Boston Children's Hospital and involving experts from the University of Aberdeen, The Institute of Cancer Research, London, and the Swiss Institute of Bioinformatics has revealed that a protein called Yap triggers the growth of a rare childhood muscle cancer, called embryonal rhabdomyosarcoma (ERMS).
The researchers are now searching for a small molecule or other biological agent that could inhibit Yap activity in human ERMS tumors, a majority of which showed enhanced Yap expression and activity. If such therapeutics can be found, they would then enter preclinical trials in mice.
The study appears in the scientific journal Cancer Cell.
Between 250-350 cases of rhabdomyosarcoma are seen each year in the United States. The disease most commonly begins as a noticeable swelling in the head, neck, arms, legs, or groin, and is treated by surgical removal of the tumor as well as chemotherapy and irradiation. More than 80% of children diagnosed with rhabdomyosarcoma survive.
“The current therapies for rhabdomyosarcoma, although relatively efficient, are very aggressive and drastically alter the quality-of-life of the children who survive,” said study first author, Annie Tremblay, PhD. “Most of the survivors suffer life-altering consequences such as loss of mobility or vision, growth impairment, and developmental problems, or the need for life-long hormone replacement therapies.”
During normal muscle development, stem cells turn into developmental muscle cells—called myoblasts—that divide, before finally fusing together to form long muscle fibers.
The Yap protein is fundamental to this process, with the myoblasts experiencing a marked increase in its activity during the division stage. Once enough myoblasts are present, Yap gets turned off which allows them to stop dividing and fuse together to form functional muscles.
Findings from the study show that in some instances of rhabdomyosarcoma, the Yap protein remains active—like the accelerator of a car being stuck.
“We discovered that in cases of the disease, excessive activity of a protein called Yap causes muscle stem cells to permanently divide instead of stopping to become normal muscle tissue, and rhabdomyosarcoma develops as a consequence,” added Tremblay, a postdoctoral fellow in Harvard University’s Department of Stem Cell and Regenerative Biology (HSCRB). “Interestingly, when we decreased Yap activity in mouse ERMS tumors, they decreased in size and started to form normal muscle fibers within the regressing tumors. This suggests that lowering YAP activity could eventually become a useful differentiation therapy approach in ERMS.”
The Yap finding, in addition to identifying a new clinical target, also provides scientists with the best animal model to date of rhabdomyosarcoma. Historically, studies of the cancer have been conducted in zebrafish, which have a shorter life span than mice, and tend to produce rhabdomyosarcoma tumors more readily. Yap allows researchers to generate rhabdomyosarcoma relatively quickly in a mammal, which means a new platform to explore questions related to the cancer.
This collaboration sprung from conversations between HSCRB Assistant Professor and Boston Children's Hospital scientist Fernando Camargo, PhD, and University of Aberdeen Senior Lecturer Henning Wackerhage, PhD, after the two met at a conference three years ago. Wackerhage connected Camargo, whose lab studies the Yap protein, to clinical scientists in other parts of Europe, which hastened the discoveries made. HSCRB Professor Amy Wagers, PhD, also contributed by sharing muscle cell transplant techniques.
“This study highlights and corroborates that Yap is a major regulator of differentiation in adult stem cells,” said Camargo, referring to work published last June in the journal Cell, which found Yap a key regulator of liver cell fate.
Wackerhage added: “Other research has shown that Yap is active in several other types of cancer including liver and skin cancers. These results could therefore be of wider significance in also enhancing our global understanding of the role of Yap in cancer.”
The study was supported by funding from Aberdeen charity, Friends of Anchor, Sarcoma UK, the Medical Research Council, Cancer Research UK, the Chris Lucas Trust, and Stand Up to Cancer-AACR innovative research programs, and the Canadian Institutes of Health Research.
Cited: Tremblay, et. al. The Hippo transducer YAP1 transforms activated satellite cells and is a potent effector of embryonal rhabdomyosarcoma formation. Cancer Cell. July 31, 2014 DOI: http://dx.doi.org/10.1016/j.ccr.2014.05.029
This story is adapted from a University of Aberdeen press release.
