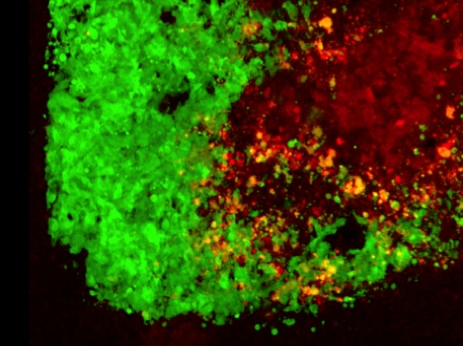
 Stem cells loaded with cancer-killing herpes virus attack a brain tumor cell. Tumor cells in green. oHSV-loaded stem cells in red. oHSV-infected tumor cells in yellow. (Credit: Khalid Shah/MGH)
Stem cells loaded with cancer-killing herpes virus attack a brain tumor cell. Tumor cells in green. oHSV-loaded stem cells in red. oHSV-infected tumor cells in yellow. (Credit: Khalid Shah/MGH)
Harvard Stem Cell Institute (HSCI) scientists at Massachusetts General Hospital have a potential solution for how to more effectively kill tumor cells using cancer-killing viruses. The investigators report that trapping virus-loaded stem cells in a gel and applying them to tumors significantly improved survival in mice with glioblastoma multiforme, the most common brain tumor in human adults and also the most difficult to treat.
The work, led by Khalid Shah, MS, PhD, an HSCI Principal Faculty member, is published in the Journal of the National Cancer Institute. Shah heads the Molecular Neurotherapy and Imaging Laboratory at Massachusetts General Hospital.
Cancer-killing or oncolytic viruses have been used in numerous phase 1 and 2 clinical trials for brain tumors but with limited success. In preclinical studies, oncolytic herpes simplex viruses seemed especially promising, as they naturally infect dividing brain cells. However, the therapy hasn’t translated as well for human patients. The problem previous researchers couldn’t overcome was how to keep the herpes viruses at the tumor site long enough to work.
Shah and his team turned to mesenchymal stem cells (MSCs)—a type of stem cell that gives rise to bone marrow tissue—which have been very attractive drug delivery vehicles because they trigger a minimal immune response and can be utilized to carry oncolytic viruses. Shah and his team loaded the herpes virus into human MSCs and injected the cells into glioblastoma tumors developed in mice. Using multiple imaging markers, it was possible to watch the virus as it passed from the stem cells to the first layer of brain tumor cells and subsequently into all of the tumor cells.
“So, how do you translate this into the clinic?” asked Shah, who also is an Associate Professor at Harvard Medical School.
“We know that 70-75 percent of glioblastoma patients undergo surgery for tumor debulking, and we have previously shown that MSCs encapsulated in biocompatible gels can be used as therapeutic agents in a mouse model that mimics this debulking,” he continued. “So, we loaded MSCs with oncolytic herpes virus and encapsulated these cells in biocompatible gels and applied the gels directly onto the adjacent tissue after debulking. We then compared the efficacy of virus-loaded, encapsulated MSCs versus direct injection of the virus into the cavity of the debulked tumors.”
Using imaging proteins to watch in real time how the virus combated the cancer, Shah’s team noticed that the gel kept the stem cells alive longer, which allowed the virus to replicate and kill any residual cancer cells that were not cut out during the debulking surgery. This translated into a higher survival rate for mice that received the gel-encapsulated stem cells.
“They survived because the virus doesn’t get washed out by the cerebrospinal fluid that fills the cavity,” Shah said. “Previous studies that have injected the virus directly into the resection cavity did not follow the fate of the virus in the cavity. However, our imaging and side-by-side comparison studies showed that the naked virus rarely infects the residual tumor cells. This could give us insight into why the results from clinical trials with oncolytic viruses alone were modest.”
The study also addressed another weakness of cancer-killing viruses, which is that not all brain tumors are susceptible to the therapy. The researchers’ solution was to engineer oncolytic herpes viruses to express an additional tumor-killing agent, called TRAIL. Again, using mouse models of glioblastoma—this time created from brain tumor cells that were resistant to the herpes virus—the therapy led to increased animal survival.
“Our approach can overcome problems associated with current clinical procedures,” Shah said. “The work will have direct implications for designing clinical trials using oncolytic viruses, not only for brain tumors, but for other solid tumors.”
Further preclinical work will be needed to use the herpes-loaded stem cells for breast, lung and skin cancer tumors that metastasize to the brain. Shah predicts the approach will enter clinical trials within the next two to three years.
This work was supported by the James S. McDonnell Foundation and the National Institutes of Health.
Cited: Duebgen, M., et. al. Stem cells loaded with multimechanistic oncolytic herpes simplex virus variants for brain tumor therapy. Journal of the National Cancer Institute. June 2014. (Early access May 16, 2014)
