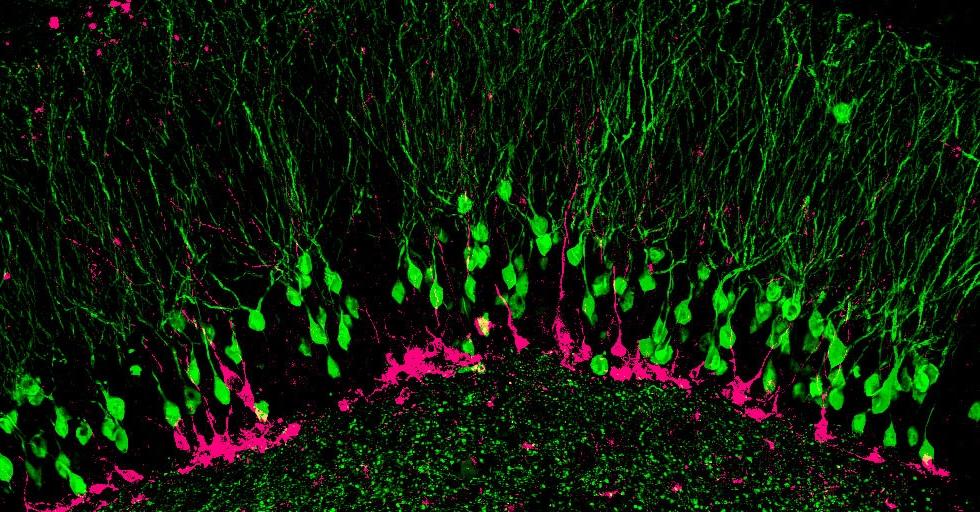
Young neurons (pink), responsible for encoding new memories, must compete with mature neurons (green) to survive and integrate into the hippocampal circuit. Photo courtesy of Kathleen McAvoy, Sahay Lab.
HSCI researchers identify new mechanisms by which new neurons sharpen memories
By Hannah L. Robbins, HSCI Communications
When it comes to the billions of neurons in your brain, what you see at birth is what get — except in the hippocampus. Buried deep underneath the folds of the cerebral cortex, neural stem cells in the hippocampus continue to generate new neurons, inciting a struggle between new and old as the new attempts to gain a foothold in the memory-forming center of the brain.
In a study published online today in Neuron, Harvard Stem Cell Institute (HSCI) researchers at Massachusetts General Hospital and the Broad Institute of MIT and Harvard in collaboration with an international team of scientists found they could bias the competition in favor of the newly generated neurons.
“The hippocampus allows us to form new memories of ‘what, when and where’ that help us navigate our lives,” said HSCI Principal Faculty member and the study’s corresponding author, Amar Sahay, PhD, “and neurogenesis—the generation of new neurons from stem cells—is critical for keeping similar memories separate.”
As the human brain matures, the connections between older neurons become stronger, more numerous, and more intertwined, making integration for the newly formed neurons more difficult. Neural stem cells become less productive, leading to a decline in neurogenesis. With fewer new neurons to help sort memories, the aging brain can become less efficient at keeping separate and faithfully retrieving memories.
The research team selectively overexpressed a transcription factor, Klf9, only in older neurons in mice, which eliminated more than one-fifth of their dendritic spines, increased the number of new neurons that integrated into the hippocampus circuitry by two-fold, and activated neural stem cells.
When the researchers returned the expression of Klf9 back to normal, the old dendritic spines reformed, restoring competition. However, the previously integrated neurons remained.
“Because we can do this reversibly, at any point in the animals life we can rejuvenate the hippocampus with extra, new, encoding units,” said Sahay, who is also an investigator with the MGH Center for Regenerative Medicine.
The authors employed a complementary strategy in which they deleted a protein important for dendritic spines, Rac1, only in the old neurons and achieved a similar outcome, increasing the survival of the new neurons.
In order to keep two similar memories separate, the hippocampus activates two different populations of neurons to encode each memory in a process called pattern separation. When there is overlap between these two populations, researchers believe it is more difficult for an individual to distinguish between two similar memories formed in two different contexts, to discriminate between a Sunday afternoon stroll through the woods from a patrol through enemy territory in a forest, for example. If the memories are encoded in overlapping populations of neurons, the hippocampus may inappropriately retrieve either. If the memories are encoded in non-overlapping populations of neurons, the hippocampus stores them separately and retrieves them only when appropriate.
Mice with increased neurogenesis had less overlap between the two populations of neurons and had more precise and stronger memories, which, according to Sahay, demonstrates improved pattern separation.
Mice with increased neurogenesis in middle age and aging cohorts exhibited better memory precision.
“We believe that by increasing the hippocampus’s ability to do what it supposed to do and not retrieve past experiences when it shouldn’t can help,” Sahay said. This may be particularly useful for individuals suffering from post-traumatic stress disorder, mild cognitive impairment, or age-related memory loss.
###
This work was done in collaboration with scientists from Harvard Stem Cell Institute, the Broad Institute of Harvard and MIT, Massachusetts General Hospital, Harvard Medical School, Columbia University, Heidelberg University, University Hospital of Cologne, Johannes Gutenberg University, and Echelon Bioscience and is supported by funding from the Ellison New Scholar in Aging; National Institute on Aging and the National Institute of Mental Health, both a part of NIH; Harvard Stem Cell Institute; and the Harvard Neurodiscovery Center/MADRC Center.
