New microgel encapsulation method paves the way for more efficient cell therapies
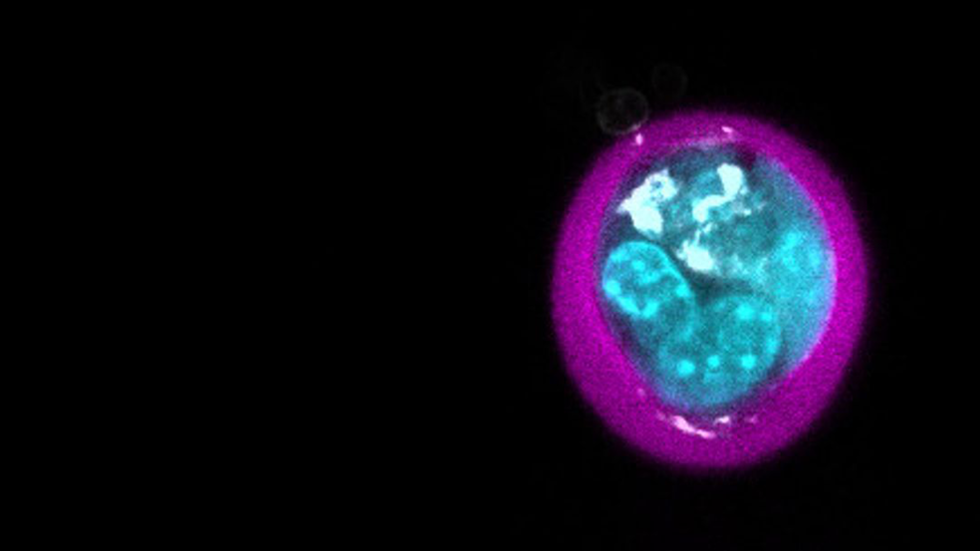
Bone marrow transplants have become a standard treatment for a range of blood cancers and diseases, but many transplants fail due to rejection by the patient’s immune system or graft-versus-host disease (in which the transplanted cells attack the patient’s healthy cells). Mesenchymal stem cells (MSCs) secrete compounds that modulate the immune system and have shown promise in mitigating these problems in animal trials. However, clinical results with MSCs have been disappointing thus far: they are rapidly cleared from the body and can draw attack from patients’ immune systems, and efforts to encapsulate MSCs in protective biomaterials have resulted in large, bulky hydrogels that cannot be given intravenously and compromise the cells’ functions.
In a scientific first, researchers from the Harvard Stem Cell Institute (HSCI), Wyss Institute for Biologically Inspired Engineering, and Harvard’s John A. Paulson School of Engineering and Applied Sciences have demonstrated that a single-cell encapsulation technology can effectively protect transplanted MSCs from clearance and immune attack and improve the success of bone marrow transplants in mice. The study, published in PNAS, includes HSCI affiliate faculty member David Mooney, Ph.D. as the senior author and HSCI co-director David Scadden, M.D. as a co-author.
“To our knowledge, this is the first example of single-cell encapsulation being used to improve cell therapies, which are becoming more widespread as treatments for a number of diseases,” said lead author Angelo Mao, Ph.D. “Our encapsulated cells can be frozen and thawed with minimal impact on the cells’ performance, which is critical in the context of hospitals and other treatment centers.”
What the researchers did
This advance builds on a method the team previously developed that uses a microfluidic device to coat individual living cells with a thin layer of an alginate-based hydrogel, creating what they term “microgels.” The process encapsulates cells with 90% efficiency, and the resulting microgels are small enough that they can be delivered intravenously. When injected into mice, MSCs encapsulated with this technique remained in the animals’ lungs ten times longer than “bare” MSCs, and remained viable for up to three days.
Because a large part of MSCs’ clinical appeal lies in their secretion of compounds that modulate the body’s immune system, the researchers needed to test how microgel encapsulation affects MSCs’ ability to function and resist immune attack. They modified their original alginate microgel by adding another compound that makes the microgel stiffer and better able to resist the body’s immune system and clearance mechanisms. They also cultured the MSCs after encapsulation to encourage them to divide and produce more cells. When these new microgels were injected into mice, their persistence increased five-fold over the previous microgel design and an order of magnitude over bare MSCs.
What the researchers found
To induce an immune response against the MSCs, the team incubated encapsulated cells in a medium containing fetal bovine serum, which is recognized by the body as foreign, before introducing them into mice. While the clearance rate of the encapsulated MSCs was higher than that observed without immune activation, it was still five times lower than that of bare MSCs. The microgels also outperformed bare MSCs when injected into mice that had a preexisting immune memory response against MSCs, which mimics human patients who are given multiple infusions of stem cells.
MSCs exposed to inflammatory cytokines respond by increasing their expression of immune-modulating genes and proteins, so the researchers next tested whether encapsulation impacted this response. They found that bare and encapsulated MSCs had comparable levels of gene expression when exposed to the same cytokines, demonstrating that the microgels did not impair MSC performance.
For their pièce de résistance, the team injected their MSC-containing microgels into mice along with transplanted bone marrow, half of which was immune-compatible with the recipient mouse and half of which was allogeneic, or an immune mismatch. Mice that received encapsulated MSCs had more than double the fraction of allogeneic bone marrow cells in their marrow and blood after nine days compared with mice that did not receive MSCs. Encapsulated MSCs also led to a greater degree of engraftment of the allogeneic cells into the host bone marrow compared to bare MSCs.
Why it matters
“One of the strong points of this work is that it uses a completely non-genetic approach to dramatically increase cell survival in transplant contexts, where it’s sorely needed,” said Mooney. “This technology nicely complements genetic engineering approaches, and in fact could be more efficient than attempting to directly modify immune cells themselves.”
Discover more
The original version of this story was published on the Wyss Institute website on July 15, 2019.
Source article: Mao, A. S. et al. (2019). Programmable microencapsulation for enhanced mesenchymal stem cell persistence and immunomodulation. PNAS. https://doi.org/10.1073/pnas.1819415116
Funding: This research was supported by the National Institutes of Health (grants R01 EB014703 and R01 EB023287) and the Wyss Institute for Biologically Inspired Engineering at Harvard University.
