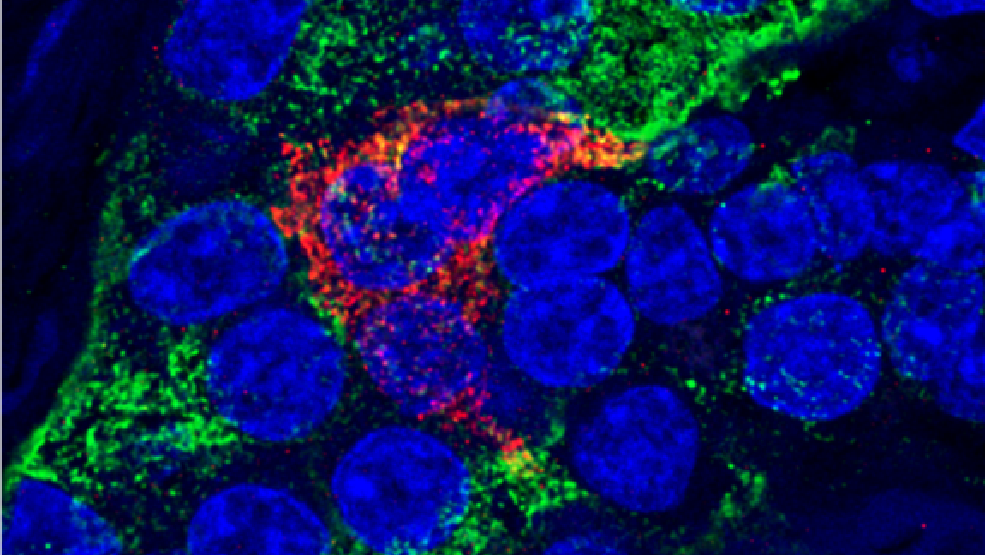
In the pancreas, insulin-producing beta cells are clustered with other hormone-producing endocrine cells and surrounded by pancreatic exocrine cells that secrete digestive enzymes. Harvard Stem Cell Institute (HSCI) researchers at the Joslin Diabetes Center now have found that an interaction between pancreatic exocrine cells and beta cells is responsible for the rare inherited disease known as mature onset diabetes of the young type 8 (MODY8). In that disease, mutated digestive enzymes are generated in pancreatic exocrine cells, which then aggregate in neighboring insulin-secreting beta cells and cause damage.
These findings may help in understanding other diseases of the pancreas, including type 1 and type 2 diabetes, in which abnormal molecular crosstalk between these two groups of cells might play a damaging role.
The study, published in the journal Nature Metabolism, was led by HSCI principal faculty member Rohit Kulkarni. He is a Joslin senior investigator, co-section head of Joslin’ Islet and Regenerative Biology Section, and a professor of medicine at Harvard Medical School.
"While the endocrine and exocrine pancreas form two distinct parts with disparate functions, their close anatomical relationship shapes their fate," said Sevim Kahraman, a postdoctoral researcher in the Kulkarni lab and first author of the study. "The pathological condition developing in one part impairs the other."
“Although MODY8 is a very rare disease, it may shed light on general mechanisms involved in the development of diabetes," said co-author Anders Molven, who is a professor at the University of Bergen in Norway. "Our findings demonstrate how a disease process that starts in the exocrine pancreas eventually may affect the insulin-producing beta-cells. We think that such negative exocrine–endocrine crosstalk could be particularly relevant for understanding some cases of type 1 diabetes.”
In MODY8, a gene mutation in exocrine cells causes the production of an abnormal digestive protein. Researchers began the study by modifying a human exocrine cell line to express that mutant protein.
When beta cells were bathed in solution from either mutated or normal exocrine cells, the beta cells took up both the mutated and normal proteins, bringing in higher numbers of the mutated proteins. The normal proteins were degraded by regular processes in the beta cells and disappeared over several hours, but the mutant proteins did not, instead forming protein aggregates. Beta cells containing the protein aggregates were impaired in secreting insulin on-demand, proliferated more slowly, and were more vulnerable to death.
Next, the researchers transplanted human exocrine cells (again expressing either the mutated or the normal digestive enzyme) along with human beta cells into a mouse model designed to accept human cells. The researchers found that the beta cells took up more of the mutated protein, which formed insoluble aggregates.
Additionally, examining pancreases from people with MODY8 who died from other causes, the investigators saw that the beta cells contained the mutated protein. "In healthy donors, we did not find even the normal protein in the beta cell," said Kulkarni.
"This MODY8 story originally started with the clinical observation of patients with diabetes also having digestive problems, which led to the finding of a common genetic denominator," said co-author Helge Raeder, a professor at the University of Bergen. "In the current study, we close the circle by mechanistically linking these clinical findings. Contrary to our expectations, a digestive enzyme normally destined for the gut was instead misled to enter the pancreatic islet in the diseased state, ultimately compromising insulin secretion."
Today, people with MODY8 are treated with insulin or oral diabetes drugs. Kulkarni and his colleagues plan to look for ways to design more tailored and personalized therapeutics. "For example, can we dissolve these protein aggregates, or limit their aggregation in the beta-cell?" said Kulkarni. "We can take cues from what has been learned in other diseases like Alzheimer's disease and Parkinson's disease that have a similar aggregation mechanism in the cells."
Read more
This story was adapted from a press release by Joslin Diabetes Center on January 17, 2022.
Kahraman, S. et al. (2022). Abnormal exocrine–endocrine cell cross-talk promotes β-cell dysfunction and loss in MODY8. Nature Metabolism. DOI: 10.1038/s42255-021-00516-2
