 A bone drug already on the market for osteoporosis may kill chronic myelogenous leukemia (CML) stem cells thought to persist in the bone marrow after standard therapy, lowering the likelihood of disease recurrence, according to a new study in mice led by researchers at the Harvard Stem Cell Institute (HSCI), the Harvard Department of Stem Cell and Regenerative Biology, and Massachusetts General Hospital.
A bone drug already on the market for osteoporosis may kill chronic myelogenous leukemia (CML) stem cells thought to persist in the bone marrow after standard therapy, lowering the likelihood of disease recurrence, according to a new study in mice led by researchers at the Harvard Stem Cell Institute (HSCI), the Harvard Department of Stem Cell and Regenerative Biology, and Massachusetts General Hospital.
The study, published in Nature Medicine on October 27, provides the first evidence in mice that altering the bone environment to make it inhospitable to leukemia stem cells can improve CML outcomes. Current CML treatments effectively target leukemia cells but not leukemia stem cells, and therefore the disease is rarely cured, the study authors said.
The research also suggests that CML and a related, more severe disease—acute myeloid leukemia (AML)—are sensitive to different changes in the bone marrow environment and may therefore require different treatment approaches.
“Traditionally, cancer therapies have always tried to target the cancer cells themselves,” said David Scadden, HSCI Co-director, Harvard Medical School (HMS) Gerald and Darlene Jordan Professor of Medicine at Mass General, and senior author of the paper. “Our work shows there might be value in targeting the cancer’s home environment as well, in combination.”
“We stepped outside the box to show it’s not all about the tumor, it’s also about where it sits,” added Daniela Krause, HMS instructor in pathology at Mass General and first author of the paper. “That hadn’t been shown before in leukemia.”
Hit them where they live
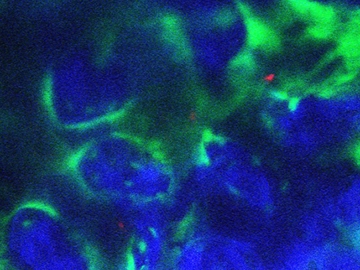 CML is a slow-progressing cancer in which leukemia stem cells in the bone marrow form excessive numbers of white blood cells called granulocytes. In addition to uncontrolled proliferation, the granulocytes themselves are abnormal.
CML is a slow-progressing cancer in which leukemia stem cells in the bone marrow form excessive numbers of white blood cells called granulocytes. In addition to uncontrolled proliferation, the granulocytes themselves are abnormal.
These “leukemia cells” crowd out healthy blood cells, leading to fatigue, infection, excess bleeding, pain, enlarged spleen, and death. CML tends to affect older adults.
Treatment advances such as the introduction of imatinib mesylate (Gleevec) have made it possible to control CML by killing leukemia cells in the blood, but most people must take the drug for the rest of their lives to prevent the cancer from returning.
Researchers suspect the reason for this is that abnormal stem cells remain in the bone marrow and produce new leukemia cells once treatment stops, like roach eggs lurking after an exterminator visit.
Scadden and team wanted to see if they could destroy those stem-cell holdouts by making their home environment inhospitable. They had a hunch about what they could try.
They turned to parathyroid hormone (PTH), which is secreted by the parathyroid glands in the neck. One of its jobs is to stimulate bone turnover—the creation and breakdown of bone cells—to release calcium into the blood. PTH and a synthetic equivalent are FDA-approved to treat osteoporosis.
Previous studies had shown that mice treated with PTH experienced altered blood cell formation in their bone marrow. Scadden and team wondered whether that meant PTH-treated mice might have an unusual response to leukemia, too.
Down to the bone
The researchers began by inducing CML in a mouse model whose bones were highly sensitive to PTH. They found that half as many of those mice developed CML compared to normal CML mouse models.
“I did the experiments, and to everyone’s surprise, the mice didn’t get leukemia,” said Krause. “I repeated the experiments and demonstrated there is a mutual interaction between the cancer and its home.”
To make sure the striking results couldn’t be traced to a quirk of the mice having been bred for PTH-sensitive bones, the researchers then treated ordinary CML mouse models with PTH. The treated mice had a 15-fold reduction in CML stem cells compared to untreated mice.
Targeting the bone was directly changing the disease outcome.
“That was a big finding, that the modulation of the environment led to the alteration of a cancer,” said Scadden, who is also professor and co-chair of Harvard University’s Department of Stem Cell and Regenerative Biology. “When you change the environment in which the cancer lives, something happens to the cancer itself.”
The team suspects the answer lies with a protein called TGF beta 1 that’s released during high bone turnover. Tests in lab dishes by another group had suggested TGF beta 1 suppresses CML cells.
“Nobody had thought about the connection between PTH effects on bone, TGF beta 1 and the impact on CML before,” said Krause.
When the researchers repeated the first test in mouse models of AML, however, the PTH-sensitive mice died faster than the non-sensitive ones. The same bone turnover protein that suppressed CML appeared to increase AML.
“This indicates that environmental niches are different for different cancers, even those as closely related as CML and AML,” said Scadden.
Hope for a cure
The team went on to show that treating CML mice with a combination of PTH and imatinib mesylate eradicated the disease more often than treating mice with imatinib mesylate only.
“This discovery raises the hope of designing agents or using PTH itself, which is already an FDA-approved drug, to influence and manipulate the bone marrow environment for CML patients and try to cure them,” said Krause.
So far, the researchers had been working with the mouse equivalent of CML. To bring the experiments closer to a human system, they injected human CML stem cells into mice whose immune systems had been suppressed so they wouldn’t reject the foreign cells. PTH treatment worked once more; it reduced the number of CML stem cells that took hold in the mice’s bone marrow.
The team is now hoping to move toward human clinical trials to assess the use of PTH or a close equivalent for treating CML.
Team members also want to investigate how they might identify different environmental niche-targeting drugs for other leukemias as well as for cancers beyond leukemia, such as breast cancer, that can be affected by hormone regulation, said Krause. She said they also would like to study whether altering the bone marrow environment can benefit people with cancers that have metastasized to the bone, such as breast and prostate cancer.
This research was supported by NIH grants R01 CA090576, R01 CA148180, R01 HL044851, R01 HL089747, K08 CA138916-02, T32 CA009216, AR060221 andR21AR060689; and by a grant from The Ellison Medical Foundation.
This story, written by Stephanie Dutchen, was provided by Harvard Medical School.
Photo: Treating mouse bone (blue) with parathyroid hormone makes the bone marrow environment inhospitable to chronic myelogenous leukemia stem cells (red) by activating bone-creating cells (green) that release the protein TGF beta 1. (Credit: Daniela Krause and Charles Lin)
