Some day, stem cells will be enlisted to help repair or replace damaged tissues and organs. They will rescue us from diseases for which drugs can only treat the symptoms. But they may have another role in our lives, one that is not so beneficial. They may in fact be the source of some, and possibly most cancers. Lurking somewhere within every tumor, some say, are a few stem cells that have lost their genetic marbles, so to speak - continuously supplying a malignant mass with cancerous cells.
Some day, stem cells will be enlisted to help repair or replace damaged tissues and organs. They will rescue us from diseases for which drugs can only treat the symptoms. But they may have another role in our lives, one that is not so beneficial. They may in fact be the source of some, and possibly most cancers. Lurking somewhere within every tumor, some say, are a few stem cells that have lost their genetic marbles, so to speak - continuously supplying a malignant mass with cancerous cells.
Stem cells are key to our normal development and health from conception through adulthood. Embryonic stem cells produce the progenitors and patterns that determine how our organs, muscles, sinews, and skeletons are formed and how they are arranged in the body. After their work is done, they leave behind a guardian population of stem cells that repair each tissue as the need arises. When the stem cell divides into two, it creates one progenitor and renews itself. The progenitor continues its path of differentiation into mature, specialized cells, while the new stem cell waits for the next round when it is called upon to replenish tissue.
Stem cells survive much longer than ordinary cells, increasing the chance that they might accumulate genetic mutations. It might take only a few mutations for one cell to lose control over its self-renewal and growth and become the source of cancer.
The idea that the remnants of our embryonic past could lead to our demise through cancer is actually a longstanding hypothesis, tracing back to 1829. Throughout the mid-19th century, theories and observations accumulated that tumors were linked to embryonal tissue growth, culminating in a comprehensive “embryonal rest” theory put forward by Julius Cohnheim in 1875. The theory stated that tumors may arise from embryonic cells left over from development, and that lie dormant until activated to become cancerous.
Today’s theories about the involvement of stem cells in cancer are really an update of the embryonal rest theory, only now we know more precisely which types of cells are involved. This advance in knowledge, more than 150 years after the theory was first proposed, came about because we now know how to identify stem cells within tumors by the protein markers on their surface.
Searching for these markers, John Dick and colleagues at the University of Toronto confirmed in 1997 that certain types of leukemia originated from a subpopulation of stem cells. In 2003, Michael Clarke of the University of Michigan and now at Stanford, found cancer stem cells in breast tumors and demonstrated that most other cells in the tumor were incapable of seeding growth on their own. Others followed with similar discoveries in brain cancer, colon cancer, bone cancer and melanoma.
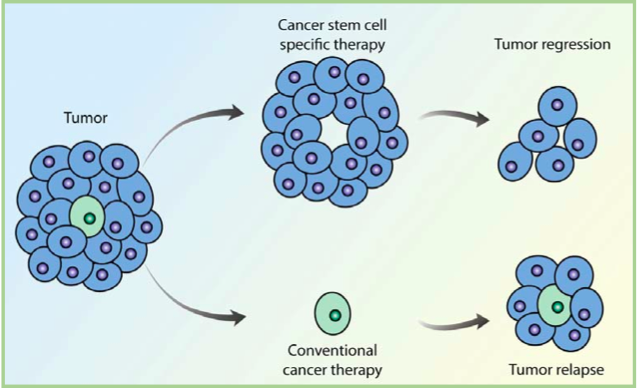
If tumors originate from just a few errant stem cells, it might explain why many treatments that reduce tumor mass fail to cure patients of cancer. Generally, these treatments target fast growing cells, which might leave the slow cycling stem cells untouched. Because they may be relatively protected from current treatment strategies, cancer stem cells are thought to be responsible for resistance to chemotherapy and the recurrence of disease.
A rationale for a new treatment strategy is emerging that specifically targets the cancer stem cells, which may only be a very small percentage of the total tumor mass. In combination with current treatments, however, these new treatments may lead to a more complete and durable response. A recent study completed by Markus Frank, Assistant Professor at Harvard Medical School, and Associate Faculty member of HSCI, identified a class of stem cells that initiate melanomas (skin cancer) in an animal model, and identified an antibody that slowed tumor growth by specifically targeting these stem cells. It was a first-time demonstration of this new therapeutic strategy.