It stands to reason that only by understanding the root causes of diseases like diabetes can we hope to develop effective therapies.
Modern biomedical research is best at finding treatments for diseases that have relatively simple causes and well-understood genetic risk factors. Unfortunately, type 1 diabetes (T1D) has very complex genetics, with many genes each making relatively small, poorly understood contributions to disease risk. Further, there are no animal models that accurately reflect the human disease. Thus, despite the expenditure of hundreds of millions of research dollars, no cures for T1D have been developed.
T1D is particularly challenging to study in human patients. By the time a patient is diagnosed with T1D, also known as juvenile diabetes, the destruction of insulin-producing beta cells by the immune system is nearly complete. Because of this, there is no way to discover what it was that led the person’s immune system to attack the beta cells in the first place. Even if it were possible to identify future T1D patients before the immune attack on beta cells began, disease onset and progression could not be studied in these individuals due to the inaccessibility of the pancreas – where beta cells are found – in a living person.
A New Model for Disease
Harvard Stem Cell Institute (HSCI) scientists are attempting an ambitious, long-term, and high-risk project to create the first animal model for T1D. Engineered mice will allow researchers to better understand the disease and increase the odds of developing effective therapies.
The Vision
Recent advances in stem cell biology have opened the door to new ways of studying T1D. Specifically, it is now possible to reprogram a skin cell from a T1D patient (or any other person) into a cell that closely resembles an embryonic stem cell. These new stem cells, called induced pluripotent stem cells (iPS cells), have the ability to give rise to any cell type in the human body. Further, iPS cells are genetically identical to the original patient, so all of the mutations that predisposed an individual to T1D are present. A virtually unlimited number of diseased cells can be produced using this method.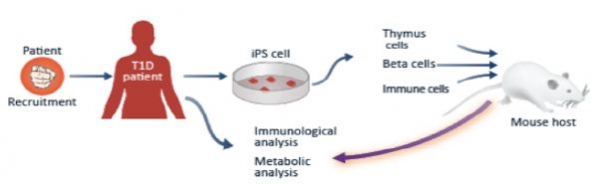
At the heart of the new T1D model will be transplanting the three human cell types that are the key components of T1D into mice. The T1D cell types are: beta cells; the immune cells that attack and destroy beta cells; and thymus cells, which educate the immune system by providing information about which cells should and should not be destroyed.
HSCI scientists will produce each of these cell types from patient-derived iPS cells while simultaneously raising genetically engineered mice that lack immune systems, allowing them to accept transplants of human cells. The researchers will transplant the T1D cell types into the mice, thereby creating a new T1D research model in which patient-derived cells can interact to generate the disease state.
A Collaborative Team
HSCI has assembled an inter-institutional team of experts in the stem cell and diabetes fields with a track record of ongoing and effective collaboration to build this model, giving us the best chance of success. Team members include Harvard Medical School, Boston Children’s Hospital, and the Immune Disease Institute.
This new T1D mouse model will allow HSCI investigators to test whether cells from a given T1D patient generate T1D in every case or whether particular environmental conditions might also be required in order to develop T1D. The model will also reveal whether all patients who present with T1D have the same disease, or whether there are different sub-types.
These are lengthy and expensive experiments and the project will take several years before major insights can be reached. But with a team of researchers now in place, even partial success would provide significant advances to biomedical research, and to the T1D community. For example, if our scientists are successful in producing beta cells from iPS cells, this would provide an unlimited source of urgently needed cells for transplantation therapy.
A new approach to T1D research is desperately needed. It makes no sense to spend any more precious research dollars on experiments that lead to insufficient results. HSCI’s project, while risky, has the potential to open up a new era in T1D research—one in which the disease is understood.
 Making Beta Cells
Making Beta Cells
HSCI scientists are also investigating how to treat diabetics with stem cell therapies. The goal is to create glucose-sensing, insulin-producing beta cells, the cells impacted in both type I and type II diabetes. The HSCI is approaching the problem in four ways.
Growing Cells
Our researchers have refined the process of making general pancreatic endocrine cells from embryonic stem cells so that it is more efficient. Using a unique bioreactor, it is possible to grow cells at a large enough scale to run extensive experiments and be effective therapeutically.
With this in place, researchers are now honing in on the final step of the process—turning pancreatic endocrine cells into beta cells. This last maturation step has taken place in the live mouse, but has not yet been accomplished in the lab setting. Once the process can be controlled, it will ensure that patients receive the right number and type of cells.
Direct Differentiation
A second approach is to turn other types of pancreatic cells into beta cells. HSCI Co-Director Douglas Melton, PhD, proved that this could be done by turning pancreatic acinar (digestive) cells in the live mouse into insulin-producing beta cells. Melton’s lab used a virus to make genetic modifications that could not be safely done in humans. However, the experiments proved that this type of “direct differentiation” was conceptually feasible. Scientists are now working with the same strategy on other closely related cell types, such as liver cells.
Reprogramming
A third approach is to take cells from patients who have diabetes, use reprogramming methods to create induced pluripotent stem cells (iPS cells) and then differentiate them into beta cells. Melton’s lab has already created many iPS cell lines from diabetics with different genetic backgrounds, but again, the final step of transforming iPS cells into beta cells is not yet ready for humans.
Self-Regulation
A fourth approach is not to turn other cells into beta cells but to get beta cells to make more of themselves. Thanks to work coming out of the Melton lab, it is now understood that beta cells in the pancreas do make more of themselves, albeit very slowly and at a low rate. This replication slows down even further with age.
These findings suggest that a possible strategy for type II diabetes and very early-diagnosed T1D is to increase the beta cell replication rate. Intrigued by the fact that the number of beta cells increases significantly in pregnant mammals, the Melton lab studied the differences in genes that are on or off before, during, and after pregnancy. The researchers identified several causal factors that led to the recent formation of a three-way joint development agreement between the lab, a biotechnology company, and a large pharmaceutical company to develop drugs that could affect this pathway. If successful, this development will result in a drug that can directly raise the number of beta cells by stimulating replication.
Disease Prevention
HSCI’s work to make functional beta cells is in motion, and while the reproducible production of beta cells from stem cells has not yet been achieved, our scientists have demonstrated that this is possible.
Encapsulation Devices
Parallel to this effort, and in planning for its success, HSCI investigators are now focusing attention on developing and testing devices for implantation, eventually in human subjects, that would protect functional beta cells from being attacked by the immune system.
Such devices would serve as receptacles for beta cells that would be transplanted into people, allowing the cells to read glucose levels and secrete insulin, while simultaneously preventing physical access by immune cells (T-cells). This is possible because glucose and insulin are both relatively small molecules, tiny in comparison to the size of T-cells.
In the longer term, HSCI researchers aim to make beta cells that are opaque or invisible to the immune attack, so that shielding them with an encapsulation device will not be necessary. But making an opaque beta cell is a complex challenge— one that is unlikely to be met in the next few years.
Encapsulation devices that have been developed to date by various labs and small manufacturers include those with alginate coating as well as devices that resemble “tea bags” made from high tech materials such as Teflon and Gore-Tex. In each of these configurations, the aim is to have a mesh or filter that allows sugar and insulin to easily pass through while blocking passage of the larger immune cells.
At this time, these devices are not routinely available, and those that have been created are expensive. A further problem that has slowed the development and testing of this approach has been the lack of a reproducible supply of human beta cells. Thus, existing devices have only been tested with human or rodent pancreatic tissue, which are inherently variable because of the state of the tissue donation and the method used for isolation. As such, there has been a distinct lack of robust testing of any device, most of which to date are high cost and in limited supply.
HSCI scientists will work to further develop and test encapsulation devices, in the first instance, using the immature beta cells that can now be derived from stem cells and mature following transplantation into animals. The focus of this work will be to explore the maturation and function of cells transplanted into devices.
